Click here for more information about how Roche is responding to COVID-19
Questions About Our Medicines? Visit medinfo.roche.com
Powerful insights to guide efficient, personalised treatment decisions
A comprehensive approach to genomic profiling
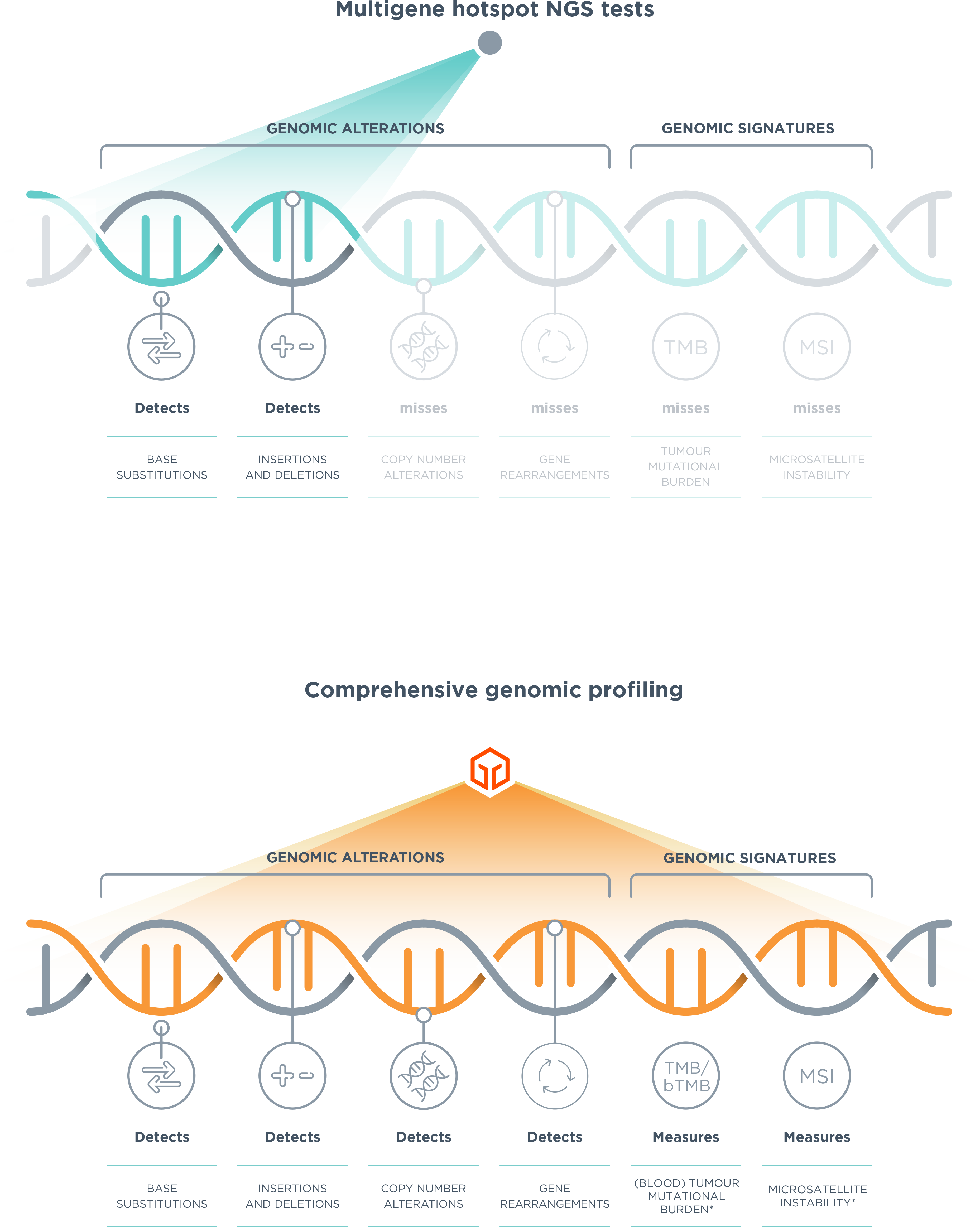
The Foundation Medicine comprehensive genomic profiling approach broadly analyses the tumour genome to identify clinically relevant genomic alterations and signatures, and potentially expands patients’ treatment options. 1,2,4–6,8–14 Clinicians then receive a clear, in-depth report that supports clinical decision-making by providing insights on the patient’s genomic profile, associated targeted therapies and immunotherapies† as well as relevant clinical trials.15

A high-quality portfolio of services
Our portfolio of extensively validated comprehensive genomic profiling services provides powerful insights to guide efficient, personalised treatment decisions – across the patient journey.1─9
Overview of services
Combined commitment
As part of our long-standing commitment to pioneering progress in precision medicine, molecular insights leader Foundation Medicine has become a member of the Roche Group. Together Roche and Foundation Medicine are bringing comprehensive genomic profiling to cancer patients around the world, combining Roche’s expertise and commitment in oncology and Foundation Medicine’s leading technology, validation and experience in cancer profiling.16*Comprehensive genomic profiling is a next-generation sequencing approach able to detect both novel and known variants, including all classes of genomic alterations (base substitutions, insertions and deletions, copy number alterations and rearrangements) and genomic signatures (for example Tumor Mutational Burden [TMB] and blood Tumour Mutational Burden [bTMB], Microsatellite Instability [MSI] or Loss of Heterozygosity [LoH]), to provide prognostic, diagnostic and predictive insights that inform treatment decisions for individual patients across all cancer types (CGP insights can be generated either from an end-to-end, curated reporting service or through in-house testing solutions).
†Approved therapies are ranked alphabetically within NCCN therapy categories (for additional information on the NCCN categories please refer to the NCCN Compendium® at www.nccn.org).
‡Clinical validation demonstrated concordance with the following companion diagnostics: cobas® EGFR Mutation Test, Ventana ALK (DSF3) CDx Assay, Vysis ALK BreakApart FISH Probe Kit, therascreen® KRAS RGQ PCR Kit, Dake HER2 FISH PharmDx® Kit, cobas® BRAF V600 Mutation Test, THxlD® BRAF kit. For more information, please see the FoundationOne®CDx Technical Specifications available at: https://www.foundationmedicine.qarad.eifu.online/foundationmedicine/en/
§Clinical validation demonstrated concordance with the following diagnostics: cobas® EGFR Mutation Test v2, a tumour tissue polymerase chain reaction-based clinical trial assay (CTA), and an externally validated circulating cell-free DNA-based next-generation sequencing assay. For more information please see the FoundationOne Liquid®CDx Technical Specifications available at: https://www.foundationmedicine.qarad.eifu.online/foundationmedicine/en/
¥TMB reported by FoundationOne CDx and FoundationOne Heme. bTMB reported by FoundationOne Liquid CDx. MSI reported by FoundationOne CDx and FoundationOne Heme, MSI-H reported by FoundationOne Liquid CDx.
bTMB, blood Tumour Mutational Burden; FDA, US Food and Drug Administration; FFPE, formalin-fixed paraffin-embedded; MSI, Microsatellite Instability; NCCN, National Comprehensive Cancer Network; NGS, next generation sequencing; LoH, Loss of Heterozygosity; TMB, Tumour Mutational Burden.
- FoundationOne®CDx Technical Specifications. Available at: https://www.foundationmedicine.qarad.eifu.online/foundationmedicine/en/foundationmedicine (Accessed August 2021).
- Frampton GM et al. Nat Biotechnol 2013; 31: 1023–1031.
- FoundationOne®CDx FDA Approval, 2017. Available at:
https://www.accessdata.fda.gov/cdrh_docs/pdf17/P170019a.pdf (Accessed August 2020). - Clark TA et al. J Mol Diagn 2018; 20: 686–702.
- FoundationOne®Liquid CDx Technical Specifications. Available at: https://www.foundationmedicine.qarad.eifu.online/foundationmedicine/en/foundationmedicine (Accessed August 2021).
- Woodhouse, R. et al. Clinical and analytical validation of FoundationOne®Liquid CDx, a novel 324-gene cfDNA-based comprehensive genomic profiling assay for cancers of solid tumor origin. PLoS One 2020; 15(9): e0237802. https://doi.org/10.1371/journal.pone.0237802.
- FoundationOne®Liquid CDx FDA Approval, 2020. Available at: www.foundationmedicine.com/press-releases/445c1f9e-6cbb-488b-84ad-5f133612b721 (Accessed August 2021).
- FoundationOne®Heme Technical Specifications. Available at: https://www.foundationmedicine.qarad.eifu.online/foundationmedicine/en/foundationmedicine (Accessed August 2021).
- He J et al. Blood 2016; 127: 3004–3014.
- Drilon A et al. Clin Cancer Res 2015; 21: 3631–3639.
- Rankin A et al. Oncologist 2016; 21: 1306–1314.
- Ross JS et al. Cancer 2016; 122: 2654–2662.
- Suh JH et al. Oncologist 2016; 21: 684–691.
- Hirshfield KM et al. Oncologist 2016; 21: 1315–1325.
- FoundationOne®CDx Sample Report. Available at: https://rochefoundationmedicine.com/F1CDxreport_TMBLung_EU (Accessed August 2021).
- Roche Media Release, 2018. Available at: https://www.roche.com/media/releases/med-cor-2018-06-19.htm (Accessed August 2021).
